Radius VSM helps clinicians monitor various physiological measurements.
Masimo has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its patient-worn, continuous multi-parameter vital signs monitor called Radius VSM. 6 Parameters Monitor
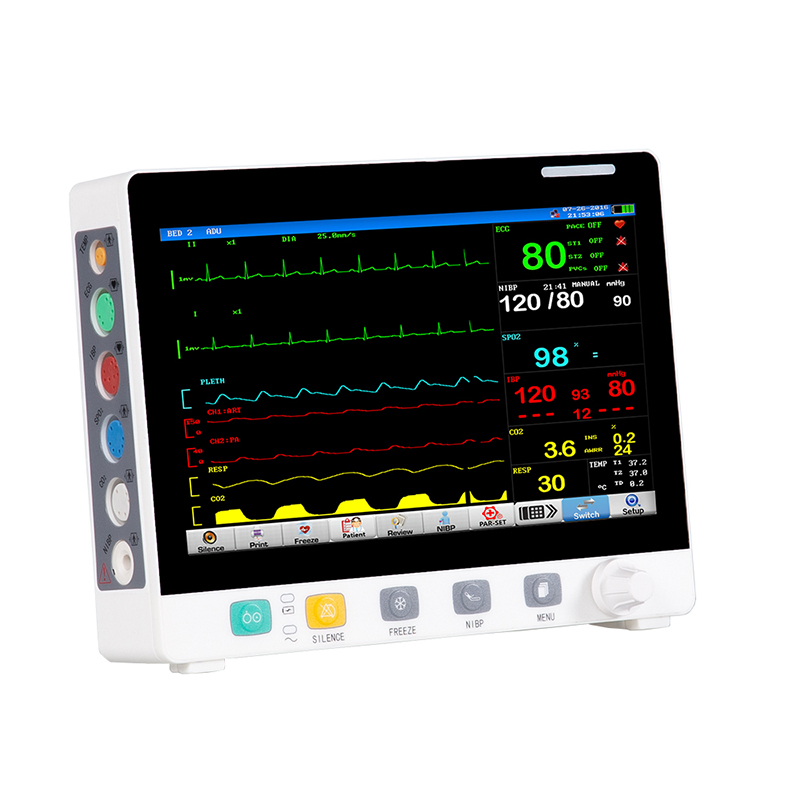
Developed using a modular platform, Radius VSM enables clinicians to monitor several physiological measurements such as Masimo SET pulse oximetry, temperature, respiration rate, electrocardiography and non-invasive blood pressure.
The device, which combines the accuracy of larger bedside monitors with the comfort of a wearable device, ensures continuous monitoring even during movement.
Masimo founder and CEO Joe Kiani said: “Radius VSM’s unique scalability, versatility, advanced connectivity and a broad range of accurate and automated continuous measurements – all in a wearable device that can be quickly and easily deployed anywhere in the hospital – make it a game-changing tool for clinicians everywhere.
“Doctors, nurses and patients in Europe are already experiencing the advantages of Radius VSM and we are excited to share them with US hospitals now too.”
Radius VSM is a modular and wearable device that enables healthcare providers to equip any hospital bed with extensive monitoring capabilities.
The device enables the rapid integration or removal of measurement technologies to suit various monitoring scenarios.
It eliminates the need for extra bedside equipment, network infrastructure or any tethered connections.
Radius VSM can function as a self-contained device or can be linked wirelessly to Masimo bedside monitors such as the Masimo Hospital Automation platform and Root.
This leads to the streamlining of clinical workflows by automating the transfer of patient-related data to remote monitoring systems such as electronic medical records and Masimo Patient SafetyNet.
7 Inch Patient Monitor The leading site for news and procurement in the medical device industry
