The list of controlled substances posted on the DHEC website is for informational purposes only. The posted list includes all substances scheduled in accordance with the provisions of SC Code Section 44-53-160, designated by the DHEC Board and transmitted to the General Assembly since the last SC Code update in 2012, as well as the list of scheduled substances currently in the SC Code for Schedules I-V. Please review the Board Orders posted on this page, as well as the Federal Register, for detailed information on the scheduling actions of controlled substances.
N,N-diethyl-2-(2-( 4-methoxybenzyl)-5-nitro-lH-benzimidazol-1-yl)ethan- l- amine Atah2-(2-Aminothiazol-4-Yl)Aceticacid
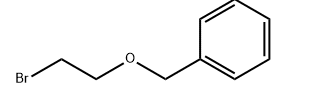
N,N-diethyl-2- (2-(4-fluorobenzyl)-5-nitro-lH-benzimidazol-1-yl)ethan-1- amine
2-(2-( 4-ethoxybenzyl)-lH-benzimidazol-1-yl)-N,N-diethylethan-1-amine
1-(4- methoxyphenyl)-N-methylpropan-2- amine (paramethoxymethamphetamine
methyl 2-(1- (4-fluorobutyl)-1H-indazole-3- carboxamido)-3,3-dimethylbutanoate

Ethyl-2-(Aminothiazol-4-Yl)-2-Oxo-Acetate(Eaoa) N-(1-(2-fluorophenethyl)piperidin-4-yl)-N-(2-fluorophenyl)propionamide (2′-fluoro ortho-fluorofentanyl; 2′-fluoro 2-fluorofentanyl