A recent study report named "Formamide Market" provides a comprehensive analysis of the market's development potential, income generating potential, and present trends. The report includes comprehensive segmentation data by type [98percent Purity , 99percent Purity], application [Htf - Pharmaceutical Processing , Polymer and Plastic , Process Solvents], and geographic location. It creates opportunities for rivals in key growth industries. The Formamide market research offers an in-depth overview of the industry for companies attempting to remain ahead of this ever changing sector. It covers growth analysis, as well as historical and predicted data on expenses, revenue, demand, and supply, making it a must-have tool for businesses of all kinds.
The study provides an in-depth examination of the market environment as well as insights into the fundamental variables impacting market growth. [94] The study report provides useful information for companies and investors wishing to take advantage of the potential of the Formamide market, from rising trends to development prospects. 3-Aminopropyldimethylamine 109-55-7
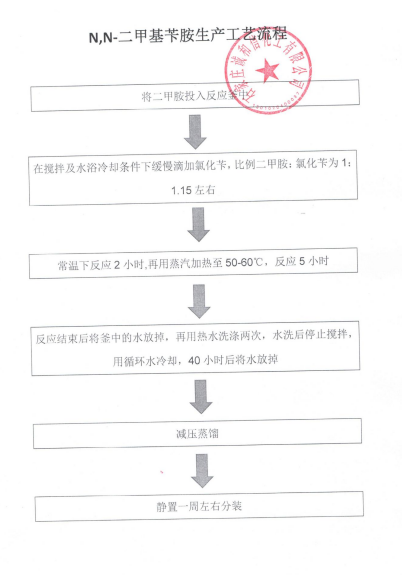
Get a Sample Copy of the Formamide Market Report [2023-2030]
The global Formamide market was valued at USD million in 2019 and it is expected to reach USD million by the end of 2026, growing at a CAGR of percent during 2021-2026.
Key Players in the Global Formamide Market Covered Are:
Eastman BASF Suqian Xinya Technology SHIJIAZHUANG SINCERE CHEMICAL Zhejiang Realsun Chemical Shandong Rongyue Chemical
Get a sample PDF of the report at: https://www.industryresearch.co/enquiry/request-sample/17128574
Introduction of the Formamide Market and Insights
The report effortlessly integrates thorough quantitative and qualitative analyses. It includes a high-level overview of the market's total size, industrial chain, and market dynamics. It also digs into micro-level data, evaluating market segmentation by kind, application, and area. As a consequence, it provides a thorough and in-depth grasp of the Formamide market, covering all critical areas and offering a full perspective of the sector.
In a word, this study is a must-read for industry participants, investors, researchers, consultants, business strategists, and anybody else who has a stake in the market or plans to enter it in any way.
The Formamide market research report is based on extensive primary and secondary research. It provides a complete analysis of the market's present and future aims, as well as a competitive study of the industry classified by application, type, and regional trends. Furthermore, the study provides a dashboard overview of the performance of the market's major firms, highlighting their past and present achievements. To give exact and complete information on the Formamide Market, the research utilizes a number of approaches and analysis.
Inquire or Share Your Questions If Any Before the Purchasing This Report: https://www.industryresearch.co/enquiry/pre-order-enquiry/17128574
Global Formamide Market is segmented into various types and applications according to product type and category. In terms of Value and Volume, the growth of the market is calculated by providing CAGR for the forecast period for years 2023 to 2030.
Based on types, the Formamide market from 2017 to 2030 is primarily split into:
Based on applications, the Formamide market from 2017 to 2030 covers:
Impact of the COVID-19 Pandemic on the Formamide Market:
The Formamide Market research examines the influence of Coronavirus (COVID-19) on the Formamide business. The impact of the COVID-19 epidemic on the industry was thoroughly detailed in the Formamide Market study. During a specific time, Formamide was fully risk assessed and industry recommendations were given. This paper also examines the markets for Pre COVID-19 and Post COVID-19. In addition, the research examines the impact of COVID-19 from the standpoint of the industry chain.
The report's main highlight is that it provides firms in the sector with a strategic study of the impact of COVID-19. At the same time, this research analysed the market of the Leading 20 nations and introduced the market potential of these countries.
TO KNOW HOW COVID-19 PANDEMIC AND RUSSIA UKRAINE WAR WILL IMPACT THIS MARKET - REQUEST A SAMPLE
The research provides a fundamental introduction to the industry, including definitions, classifications, and the industry chain's structure. It analyses the worldwide Formamide market, analyzing development trends, the competitive landscape, and the development status of major regions. The paper also goes into development policies, goals, manufacturing methods, and cost structures. It gives an in-depth analysis of import/export consumption, supply and demand dynamics, pricing, revenue, and gross margins. The study focuses on the key players in the market, including information such as company profiles, product photos and specifications, shipments, price, revenue, and contact information. Furthermore, the paper examines the changing trends in the Formamide sector.
The Formamide market report provides a detailed analysis of global market size, regional and country-level market size, segmentation market growth, market share, competitive landscape, sales analysis, the impact of domestic and global market players, value chain optimization, trade regulations, recent developments, opportunities analysis, strategic market growth analysis, product launches, area marketplace expanding, and technological innovations during the forecast period (2023-2030).
Some of the key questions answered in this report:
Get a Sample Copy of the Formamide Market Report 2023-2030
The Formamide market report includes a descriptive overview of Enterprise Videos, covering their applications, advantages, limitations, and more. In addition, the report provides an extensive account of the currently available Formamide that will impact the future market of Enterprise Videos.
The report contains a detailed review of the Formamide market, encompassing historical and forecasted market size. This information will provide an edge for developing business strategies by understanding the trends shaping and driving the Formamide market.
The Global Formamide Market (2023-2030) report offers an in-depth analysis of the global Formamide market, including a detailed description of market sizing and growth. The report analyzes the global Formamide market by value and region, providing regional analysis for various regions such as the US, Europe, Japan, China, and India.
Moreover, the report assesses the key opportunities in the market and outlines the factors driving the growth of the industry. The report forecasts the growth of the overall global Formamide market for the period 2023-2030, taking into consideration the previous growth patterns, growth drivers, and current and future trends.
Regions and Countries Level Analysis
Regional analysis is another highly comprehensive part of the research and analysis of the global Formamide market presented in the report. This section sheds light on the sales growth of different regional and country-level Formamide markets. The research report provides a detailed and accurate country-wise volume analysis and region-wise market size analysis of the global Formamide market.
Geographically, the report includes research on production, consumption, revenue, market share, and growth rate, and forecast (2017-2030) of the following regions:
Reasons to Buy this Formamide Market Research Report-
Comprehensive Coverage- Our report provides a descriptive overview of Enterprise Videos, including their applications, advantages, and limitations. It also covers historical and forecasted market size, providing an edge for developing effective business strategies.
In-depth Analysis- The report offers an extensive account of the currently available Enterprise Video, assessing key opportunities and outlining the factors driving the growth of the industry. It also provides a detailed analysis of the global Formamide market by value and region, including regional analysis for various regions such as the US, Europe, Japan, China, and India.
Timely Insights- The report takes into consideration the impact of the COVID-19 pandemic and the Russia-Ukraine conflict on the Formamide industry, providing a timely understanding of the latest market trends and future growth potential.
Marketing Advantage- By leveraging our report's insights, you can gain a marketing advantage by understanding the trends shaping and driving the Formamide market. This knowledge can help you position your business strategy to capitalize on the opportunities presented by the Formamide industry.
Trusted Source- Our report is based on extensive research and analysis, and our team of experts has a proven track record of delivering reliable and accurate market insights. By purchasing our report, you can be confident that you are getting the most up-to-date and trustworthy information available.
Purchase this report (Price 2900 USD for single user license) -https://www.industryresearch.co/purchase/17128574
1Formamide MarketOverview 1.1Formamide ProductScope 1.2Formamide SegmentbyType 1.2.1GlobalFormamide SalesbyType(2018and2022and2029) 1.3Formamide SegmentbyApplication 1.4Formamide MarketEstimatesandForecasts(2018-2029) 1.4.1GlobalFormamide MarketSizeinValueGrowthRate(2018-2029) 1.4.2GlobalFormamide MarketSizeinVolumeGrowthRate(2018-2029) 1.4.3GlobalFormamide PriceTrends(2018-2029) 2Formamide EstimatesandForecastsbyRegion 2.1GlobalFormamide MarketSizebyRegion:2018VS2022VS2029 2.2GlobalFormamide RetrospectiveMarketScenariobyRegion(2018-2023) 2.2.1GlobalFormamide SalesMarketSharebyRegion(2018-2023) 2.2.2GlobalFormamide RevenueMarketSharebyRegion(2018-2023) 2.3GlobalFormamide MarketEstimatesandForecastsbyRegion(2024-2029) 2.3.1GlobalFormamide SalesEstimatesandForecastsbyRegion(2024-2029) 2.3.2GlobalFormamide RevenueForecastbyRegion(2024-2029) 2.4GeographicMarketAnalysis:MarketFactsandFigures 2.4.1UnitedStatesFormamide EstimatesandProjections(2018-2029) 2.4.2EuropeFormamide EstimatesandProjections(2018-2029) 2.4.3ChinaFormamide EstimatesandProjections(2018-2029) 2.4.4JapanFormamide EstimatesandProjections(2018-2029) 2.4.5SoutheastAsiaFormamide EstimatesandProjections(2018-2029) 2.4.6IndiaFormamide EstimatesandProjections(2018-2029) 3GlobalFormamide CompetitionLandscapebyPlayers 3.1GlobalTopFormamide PlayersbySales(2018-2023) 3.2GlobalTopFormamide PlayersbyRevenue(2018-2023) 3.3GlobalFormamide MarketSharebyCompanyType(Tier1,Tier2andTier3)and(basedontheRevenueinFormamide asof2022) 3.4GlobalFormamide AveragePricebyCompany(2018-2023) 3.5ManufacturersFormamide ManufacturingSites,AreaServed,ProductType 3.6ManufacturersMergersandAcquisitions,ExpansionPlans 4GlobalFormamide MarketSizebyType 4.1GlobalFormamide HistoricMarketReviewbyType(2018-2023) 4.1.1GlobalFormamide SalesbyType(2018-2023) 4.1.2GlobalFormamide RevenuebyType(2018-2023) 4.1.3GlobalFormamide PricebyType(2018-2023) 4.2GlobalFormamide MarketEstimatesandForecastsbyType(2024-2029) 4.2.1GlobalFormamide SalesForecastbyType(2024-2029) 4.2.2GlobalFormamide RevenueForecastbyType(2024-2029) 4.2.3GlobalFormamide PriceForecastbyType(2024-2029) 5GlobalFormamide MarketSizebyApplication 5.1GlobalFormamide HistoricMarketReviewbyApplication(2018-2023) 5.1.1GlobalFormamide SalesbyApplication(2018-2023) 5.1.2GlobalFormamide RevenuebyApplication(2018-2023) 5.1.3GlobalFormamide PricebyApplication(2018-2023) 5.2GlobalFormamide MarketEstimatesandForecastsbyApplication(2024-2029) 5.2.1GlobalFormamide SalesForecastbyApplication(2024-2029) 5.2.2GlobalFormamide RevenueForecastbyApplication(2024-2029) 5.2.3GlobalFormamide PriceForecastbyApplication(2024-2029) 6UnitedStatesFormamide MarketFactsandFigures 6.1UnitedStatesFormamide SalesbyCompany 6.1.1UnitedStatesFormamide SalesbyCompany(2018-2023) 6.1.2UnitedStatesFormamide RevenuebyCompany(2018-2023) 6.2UnitedStatesFormamide SalesBreakdownbyType 6.2.1UnitedStatesFormamide SalesBreakdownbyType(2018-2023) 6.2.2UnitedStatesFormamide SalesBreakdownbyType(2024-2029) 6.3UnitedStatesFormamide SalesBreakdownbyApplication 6.3.1UnitedStatesFormamide SalesBreakdownbyApplication(2018-2023) 6.3.2UnitedStatesFormamide SalesBreakdownbyApplication(2024-2029) 7EuropeFormamide MarketFactsandFigures 7.1EuropeFormamide SalesbyCompany 7.1.1EuropeFormamide SalesbyCompany(2018-2023) 7.1.2EuropeFormamide RevenuebyCompany(2018-2023) 7.2EuropeFormamide SalesBreakdownbyType 7.2.1EuropeFormamide SalesBreakdownbyType(2018-2023) 7.2.2EuropeFormamide SalesBreakdownbyType(2024-2029) 7.3EuropeFormamide SalesBreakdownbyApplication 7.3.1EuropeFormamide SalesBreakdownbyApplication(2018-2023) 7.3.2EuropeFormamide SalesBreakdownbyApplication(2024-2029) 8ChinaFormamide MarketFactsandFigures 8.1ChinaFormamide SalesbyCompany 8.1.1ChinaFormamide SalesbyCompany(2018-2023) 8.1.2ChinaFormamide RevenuebyCompany(2018-2023) 8.2ChinaFormamide SalesBreakdownbyType 8.2.1ChinaFormamide SalesBreakdownbyType(2018-2023) 8.2.2ChinaFormamide SalesBreakdownbyType(2024-2029) 8.3ChinaFormamide SalesBreakdownbyApplication 8.3.1ChinaFormamide SalesBreakdownbyApplication(2018-2023) 8.3.2ChinaFormamide SalesBreakdownbyApplication(2024-2029) 9JapanFormamide MarketFactsandFigures 9.1JapanFormamide SalesbyCompany 9.1.1JapanFormamide SalesbyCompany(2018-2023) 9.1.2JapanFormamide RevenuebyCompany(2018-2023) 9.2JapanFormamide SalesBreakdownbyType 9.2.1JapanFormamide SalesBreakdownbyType(2018-2023) 9.2.2JapanFormamide SalesBreakdownbyType(2024-2029) 9.3JapanFormamide SalesBreakdownbyApplication 9.3.1JapanFormamide SalesBreakdownbyApplication(2018-2023) 9.3.2JapanFormamide SalesBreakdownbyApplication(2024-2029) 10SoutheastAsiaFormamide MarketFactsandFigures 10.1SoutheastAsiaFormamide SalesbyCompany 10.1.1SoutheastAsiaFormamide SalesbyCompany(2018-2023) 10.1.2SoutheastAsiaFormamide RevenuebyCompany(2018-2023) 10.2SoutheastAsiaFormamide SalesBreakdownbyType 10.2.1SoutheastAsiaFormamide SalesBreakdownbyType(2018-2023) 10.2.2SoutheastAsiaFormamide SalesBreakdownbyType(2024-2029) 10.3SoutheastAsiaFormamide SalesBreakdownbyApplication 10.3.1SoutheastAsiaFormamide SalesBreakdownbyApplication(2018-2023) 10.3.2SoutheastAsiaFormamide SalesBreakdownbyApplication(2024-2029) 11IndiaFormamide MarketFactsandFigures 11.1IndiaFormamide SalesbyCompany 11.1.1IndiaFormamide SalesbyCompany(2018-2023) 11.1.2IndiaFormamide RevenuebyCompany(2018-2023) 11.2IndiaFormamide SalesBreakdownbyType 11.2.1IndiaFormamide SalesBreakdownbyType(2018-2023) 11.2.2IndiaFormamide SalesBreakdownbyType(2024-2029) 11.3IndiaFormamide SalesBreakdownbyApplication 11.3.1IndiaFormamide SalesBreakdownbyApplication(2018-2023) 11.3.2IndiaFormamide SalesBreakdownbyApplication(2024-2029) 12CompanyProfilesandKeyFiguresinFormamide Business 13Formamide ManufacturingCostAnalysis 14MarketingChannel,DistributorsandCustomers 14.1MarketingChannel 14.2Formamide DistributorsList 14.3Formamide Customers 15MarketDynamics 15.1Formamide IndustryTrends 15.2Formamide MarketDrivers 15.3Formamide MarketChallenges 15.4Formamide MarketRestraints 16ResearchFindingsandConclusion 17Appendix 17.1ResearchMethodology 17.1.1Methodology/ResearchApproach 17.1.2DataSource 17.2AuthorList 17.3Disclaimer
For More About TOC:https://www.industryresearch.co/TOC/171285747#Tables
Industry Research Phone:US +1 424 253 0807 UK +44 203 239 8187 Email:sales@industryresearch.co Web:https://www.industryresearch.co
Press Release Distributed by The Express Wire
To view the original version on The Express Wire visit Formamide Market | New Invention and Future Demand Dynamics By 2030

Polyurethane catalyst A33 © 2023 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.