Patients who use drugs need more opportunities for HIV and hepatitis C virus (HCV) testing, as testing rates for these conditions during inpatient encounters are suboptimal among this population. These study results were published in Open Forum Infectious Diseases.
Researchers conducted a study using data sourced from 11 hospital sites in the United States to determine whether patients with a history of drug use are sufficiently screened for HIV and HCV during inpatient encounters. The study was performed from January 2020 to April 2022 and the unit of analysis was inpatient hospitalization rather than the individual patient. Information collected from each study site included total number of hospitalizations, HIV antigen/antibody test completions and positive test counts, HCV antibody test completions and positive test counts, and HCV viral load test completions. duo dengue ag igg igm rapid test
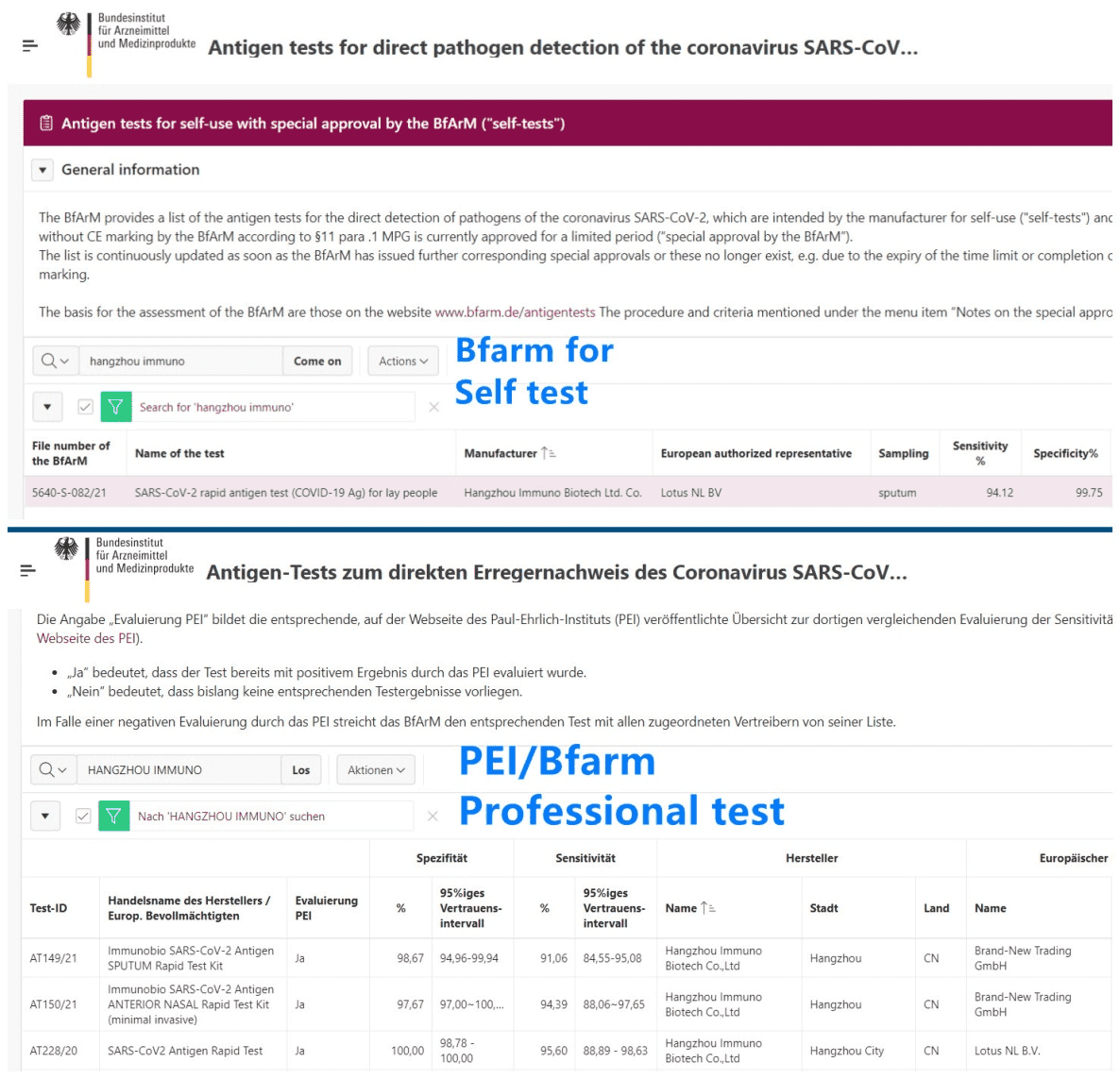
The researchers calculated testing rates and positivity rates at each site, with total number of hospitalizations used as the denominator. Difference in means They also assessed the effect of state-mandated consent requirements on testing rates and used Student t-testing to compare testing rates across sites by individual testing policies.
The final analysis comprised 65,276 inpatient encounters among patients with a history of drug use.
Across all sites, the mean rate of HIV and HCV antibody screening was 40% and 32%, respectively. The researchers noted widespread heterogeneity in the rate of testing for both HIV infection (SD, 23%) and HCV infection (SD, 15%).
Further analysis showed HIV testing rates were lower at sites with vs without verbal consent requirements (mean, 36% vs 44%; P =.607) and higher at sites with vs without opt-out policies (mean, 47% vs 32%; P =.319). They also observed no significant difference in HCV viral load testing rates between sites with vs without reflex HCV antibody testing protocols.
Overall, the mean rate of positivity was 2.9% for HIV testing and 41% for HCV antibody testing, with negative correlations between test positivity and antibody testing rates observed for both conditions (-0.177 and -0.340, respectively).
Limitations of this study include the potential lack of generalizability to other US hospitals, particularly those in rural areas. Other limitations include potential disruptions in clinical testing protocols related to the COVID-19 pandemic.
According to the researchers, “Testing PWUD [people who use drugs] during inpatient admissions is a missed opportunity to link this marginalized population to care and prevent comorbidities and new infections.”
Westgard LK, Sato T, Bradford WS, et al. National HIV and HCV screening rates for hospitalized people who use drugs are suboptimal and heterogeneous across 11 U.S. hospitals. Open Forum Infect Dis. Published online April 16, 2024. doi:10.1093/ofid/ofae204
Latest News Your top articles for Friday
Haymarket Medical Network Top Picks
Continuing Medical Education (CME/CE) Courses
Please login or register first to view this content.
rapid antibody Copyright © 2024 Haymarket Media, Inc. All Rights Reserved. This material may not be published, broadcast, rewritten or redistributed in any form without prior authorization. Your use of this website constitutes acceptance of Haymarket Media’s Privacy Policy and Terms & Conditions.
