888-776-0942 from 8 AM - 10 PM ET
SAN JOSE, Calif. , May 17, 2024 /PRNewswire/ -- Meditrina, a key leader in women's health medical devices, is thrilled to announce the FDA 510(k) clearance of its Gen 2 bipolar RF hysteroscopy system. This new system features bipolar radiofrequency technology, and a new bipolar RF device called the Aveta Glo, representing a significant advancement in minimally invasive gynecologic procedures. Trolley Type Colposcope For Gynecology
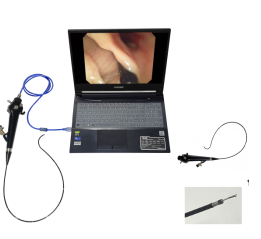
The Aveta System 2.0 builds on the success of its first-generation system, introduced in 2019, allowing physicians full control of procedures from the hysteroscope handle with integrated resection and fluid management. The system is complimented by several pathology-optimized resection devices to treat soft to calcified intrauterine pathologies resulting in 10-minute1 faster operating room turnover and significantly faster resection time improving the procedural and facility efficiency compared to existing modalities. The new System's bipolar radiofrequency technology allows for more controlled and efficient tissue removal with hemostasis, potentially improving patient outcomes. Additionally, the new bipolar RF device offers enhanced versatility, enabling physicians to perform a wider range of diagnostic and therapeutic procedures including the ability to address fibroids with deeper myometrial wall penetration across any density tissue type and provide hemostasis, when needed, expanding Meditrina's market opportunity an additional $1.5B .
"The clearance of our Gen 2 system marks a significant milestone for Meditrina and underscores our commitment to advancing women's health through innovative medical technologies," said Csaba Truckai, CEO of Meditrina. "We are dedicated to providing healthcare professionals with the tools they need to deliver the highest standard of care, and our new system exemplifies this mission by offering unparalleled performance."
Meditrina remains committed to providing comprehensive training and support for healthcare professionals to ensure the seamless integration of the Aveta System into clinical practices. This commitment reflects the company's dedication to empowering medical professionals with the tools they need to deliver optimal patient care.
In tandem with this regulatory achievement, Meditrina is also excited to announce the completion of the last $5M tranche of the $77M series C investment led by Deerfield Management Company, ShangBay Capital and other insiders. The investment is combined with an additional $5 million debt financing by SLR Capital Partners. The company is already approaching to be cash flow positive, and the additional funds will accelerate new product introduction and expansion of the sales force. This successful financing effort underscores strong investor confidence in the company's vision and growth trajectory.
"We are immensely grateful for the continued support of our investors," said Csaba Truckai, CEO of Meditrina. "This financing not only validates our strategic direction but also provides the necessary resources to scale our operations and bring our innovative solutions to a global market. We are poised for substantial growth and are confident in our ability to achieve cash flow positivity soon."
Meditrina will continue striving to better women's health, with a focus on delivering cutting-edge medical devices that enhance the quality of care for patients worldwide. Aveta System 2.0 is set to redefine standards in the field, providing healthcare professionals with advanced tools to improve outcomes and patient experiences.
About Meditrina: Founded in 2016, Meditrina, Inc. designs and develops innovative medical devices for minimally invasive gynecology. For more information, visit www.avetasystem.com.
1 Data on file at Meditrina inc.; survey conducted Oct 2019-Aug 2020 of n56 OB/GYN users and n38 Nurse users.
Contact: Abbey Taylor Associate VP of Marketing [email protected]
Meditrina, a leading innovator in gynecologic medical devices, announces the successful receipt of UKCA Mark, and CE Mark approval in accordance with ...

Duodenoscope Meditrina, a leader in gynecologic medical devices, announced today the expansion of its leadership team with the hiring of Ray Gerena, as Senior...